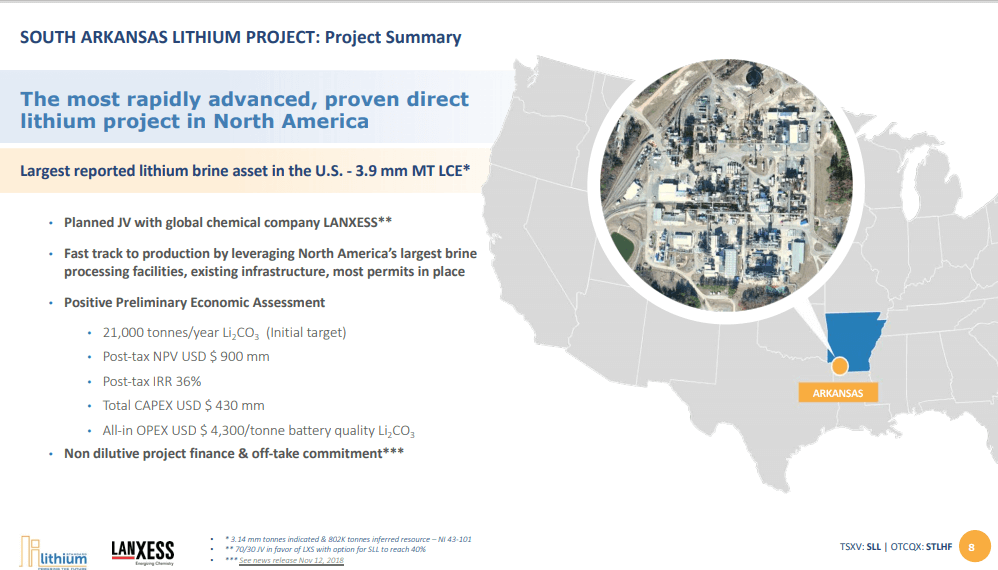
Standard Lithium (SLL) - Direct Extraction with Multi Billion Partner

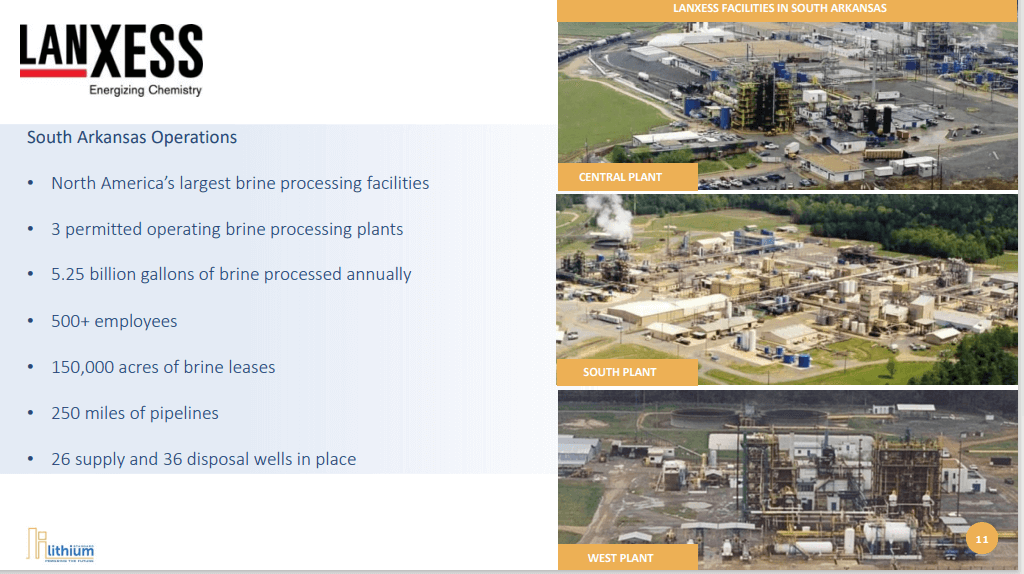
Standard Lithium's Lanxess lithium project in El Dorado, Arkansas will be a joint venture with Lanxess, who operates three bromine plants in El Dorado.
Standard Lithium CEO Robert Mintak gives an update on their Lanxess lithium project in El Dorado, Arkansas. The project will be a joint venture with German chemical company Lanxess, who currently operates three bromine plants in El Dorado.
Standard Lithium’s plan is to start with what’s already there: in this case, bromine waste brine. They’ve developed a proprietary direct lithium extraction (DLE) technology and plan to use it to extract lithium from waste brine left over from Lanxess’s bromine operations. Arkansas is an advantageous place to launch their technology. Bromine extraction has been ongoing for 60 years, so the regulatory environment is well-established. Water is plentiful and power is cheap (CAD$0.05/kw). Access is easy to local trained workers and chemical reagents, many of which are made in the same ZIP code as the bromine plants. And the waste brine reinjection process–which occurs after lithium has been extracted–is well known.
Once their technology is proven, Standard Lithium will decouple from the bromine process. Decoupling allows lithium brine extraction from areas where bromine has been depleted or where lithium is the primary resource. The direct lithium extraction technology is adaptable, with the potential to extract lithium carbonate, lithium chloride, lithium hydroxide or lithium metal, depending on current commodity prices. Decoupling will improve project economics and allow Standard Lithium to produce lithium according to demand. The market for lithium is in place as well. Lithium is processed for batteries in the United States, so the produced material doesn’t have to be exported. Additionally, the US-Mexico-Canada Free-Trade Agreement dictates that 75% of automobile components must be domestic. This includes batteries for electric vehicles, which require lithium.
The joint venture is a 70:30 relationship, with 70% held by Lanxess and 30% held by Standard Lithium. Standard Lithium has the potential to incrementally increase their ownership to 40%. They bring the intellectual property, the investment on the extraction technology and an additional 30,000 gross acres of undeveloped leases about 40 miles west of the existing El Dorado operations. Standard Lithium is responsible for all operational and staff costs, while Lanxess will do the heavy lifting. Lanxess will build commercial plants and bring initial project finance, in addition to the existing 150,000 acres of permitted leases, five billion gallons of brine flow and three operating chemical plants. Initially, Lanxess will be a 100% offtaker and convert the extracted lithium to a higher-value product, LiPF6 (lithium hexafluorophosphate).
Standard Lithium’s 2019 PEA only considers the low hanging fruit – the bromine waste brine. The PEA is based on a battery-quality lithium carbonate price of CAD$13,500/ton. Lithium carbonate prices are currently lower, but Standard Lithium is confident that prices will increase to more than CAD$10,000/t within the next two years. The CapEx of CAD$200M is significantly less than the CAD$500M CapEx required for other lithium brine operations. While Standard Lithium is waiting for Lanxess to make a final investment decision, they believe the economics are favorable and that the project will be successful and profitable.
Lanxess Project Overview
Lithium is a soft metallic element with a variety of commercial uses: glass and ceramics manufacturing, pharmaceuticals, and batteries. As global demand for rechargeable batteries grows, so does the demand for lithium. Lithium is primarily mined from 2 styles of deposits: hard rock and brine. Hard rock deposits, like the Greenbushes mine in Australia, are pegmatites (coarse-grained igneous rocks). Lithium is extracted from the mineral spodumene and refined to produce lithium hydroxide. Brine deposits are accumulations of saline groundwater enriched in lithium. They are found in arid regions like the western United States, and the Andean deserts in South America. Evaporation ponds are used to extract lithium from the brine. Brine is pumped to the surface and stored in artificial ponds, where the dry and sunny climate evaporates water. The lithium-enriched solution is then processed to produce lithium carbonate and lithium metal. However, lithium extraction via solar evaporation is a lengthy process and requires specific climate and meteorological conditions.

Lithium is also found in oilfield brines, notably those in the Smackover Formation in El Dorado, Arkansas. The Smackover was originally drilled for oil and the produced brine was disposed of, until chemists discovered that the brine is enriched in bromine. Commercial bromine production from Smackover brines has been ongoing since 1957. In addition to bromine, Smackover brines contain lithium–and Standard Lithium wants to extract it.
Standard Lithium approaches lithium mining from a different angle. They consider themselves a technology company, working backwards from the lithium deposit and developing a better way to extract the resource. At the Lanxess project, they integrate their proprietary extraction technology with existing infrastructure to recover high-purity lithium compounds in a scalable way.
Proprietary Technology
Direct lithium extraction is a modern alternative to solar brine evaporation. Traditional brine evaporation has a large geographic footprint–evaporation ponds can occupy thousands of acres. The evaporation process can take over a year and leaves behind huge salt tailings piles. Evaporation is inefficient, recovering only 50% of the dissolved lithium.
Direct lithium extraction has been used for years in humid climates like southern Arkansas. In 1978, Dow Chemical developed and patented the process of extracting bromine from brine in El Dorado. Brine is pumped to the surface and run through an exchange or solvent action process that removes lithium ions. Polishing then produces the lithium chloride. Over the past 40 years, the process has been studied extensively by university researchers and global chemical companies. Livent uses a hybrid version of direct lithium extraction in Argentina, but Standard Lithium is the first to employ this technology on its own. It’s ideal for humid climates like Arkansas, where solar evaporation simply won’t work.
Standard Lithium believes in tailoring the extraction process to each project. Each lithium brine has a different chemistry and temperature. Water and chemical reagent access and electricity costs differ for each project. This is why they developed their proprietary LiSTR technology for the Arkansas brine. LiSTR stands for Lithium Stirred Tank Reactor, a process uniquely suited to extracting lithium from bromine tail (or waste) brine. Tail brine is the brine that is left behind after bromine has been extracted. The LiSTR process uses a solid ceramic absorbent material with a crystal lattice that selectively pulls lithium ios from the tail brine. Tail brine is contained in stirred-tank reactors with the ceramic adsorbent materials mounted inside. The ceramic adsorbent material then releases the lithium for recovery.
The LiSTR process uses the bromine-extraction step to speed up lithium extraction. After bromine extraction, the brine remains heated to approximately 70°C. No additional energy is required to heat the brine and the reaction kinetics conditions for adsorption are already in place. In this process, lithium can be extracted in a matter of hours, as opposed to the months required by solar evaporation. The lithium produced from LiSTR is capable of producing a high-purity lithium chlorine solution suitable for further processing in the battery industry.
Standard Lithium capitalized on the existing bromine brine production in Arkansas to develop the LiSTR technology. Waste brine from bromine production was readily available for Standard Lithium to test at their research facility in Canada. They tested various direct lithium extraction processes from vendors and public data. Additionally, information about chemical reagents, water access, power costs and brine reinjection was easily accessible. Instead of spending millions of dollars developing the lithium resource and doing pump tests, they dedicated that money directly to building proprietary technology.
Lithium is extracted from brines in the form of lithium chloride and needs to be converted to lithium carbonate to be used in batteries. This is done using a classic carbonation operation with an established OEM. The produced lithium carbonate can be converted to another form–lithium hexafluorophosphate (LiPF6)–which goes into electrolytes for batteries. However, demand for lithium hydroxide is growing and may exceed the current demand for lithium carbonate. Electric vehicle manufacturer Tesla is driving some of this demand, having recently signed a deal with Piedmont Lithium to produce lithium hydroxide from Piedmont’s mine in North Carolina.
Standard Lithium has developed a proprietary crystallization technology to prepare lithium ions for use in batteries. Their crystallization technology is called SIFT–Selective Ion Filtration Technology. SIFT is designed to produce next generation battery-quality lithium carbonate. This technology is still in the pilot phase at Standard Lithium’s pilot plant in Vancouver. Standard Lithium is also testing the classic lithium carbonation circuit method with a partner water treatment company. Once enough data is available, they will evaluate both technologies and choose the one best suited for the Lanxess project.
Although Standard Lithium is starting with the conventional lithium conversion methods, they have their eyes set on the future. There is potential for Lanxess to produce additional forms of lithium, from lithium carbonate to lithium chloride to lithium hydroxide to lithium metals. Converting lithium chloride to lithium hydroxide with an electric cell produces large amounts of chlorine gas, which can be harmful to the environment. However, chlorine is an essential reagent in the bromine extraction process. The produced chlorine gas can be collected and repurposed, driving down the chemical reagent costs of the bromine business.
Just last week they announced that they were able to produce battery-quality lithium carbonate from lithium chloride produced at their pilot plant–bringing them one step closer Lanxess’s acceptance of the proof of concept. But as Standard Lithium CEO Robert Mintak says, they’re going to walk before they run and start with the least risky extraction and production methods.
To find out more, go to Standard Lithium's website.
Analyst's Notes




Subscribe to Our Channel
Stay Informed











